World first COVID-19 vaccine booster study launches in NHS Greater Glasgow and Clyde
20th May 2021
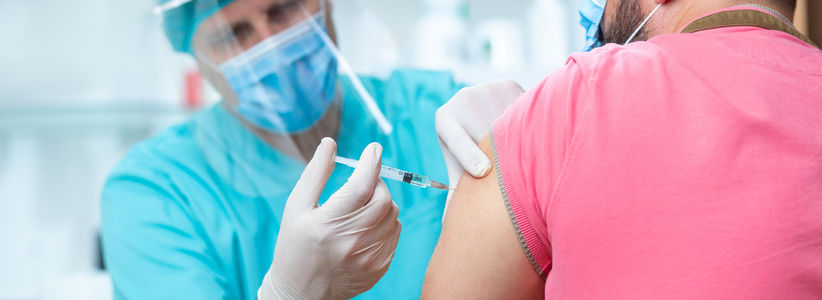
Volunteers from NHS Greater Glasgow and Clyde will soon be able to receive a third ‘booster’ COVID-19 vaccine through a new clinical trial launching this week
The Cov-Boost study, backed by £19.3 million of government funding through the Vaccines Taskforce, will be run at Glasgow Clinical Research Facilities – one of 16 sites across the UK. It will be the first in the world to provide vital data on the impact of a third dose on patients’ immune responses.
It will give scientists from around the world and the experts behind the UK’s COVID-19 vaccination programme a better idea of how effective a booster of each vaccine is in protecting the individual from the virus.
The initial findings, expected in September, will help inform decisions by the Joint Committee on Vaccination and Immunisation (JCVI) on any potential booster programme from autumn this year, ensuring the country’s most vulnerable are given the strongest possible protection over the winter period.
The trial will look at seven different COVID-19 vaccines as potential boosters, given at least 10 to 12 weeks after a second dose as part of the ongoing vaccination programme. One booster will be provided to each volunteer and could be a different brand to the one they were originally vaccinated with. Vaccines being trialled include Oxford/AstraZeneca, Pfizer/BioNTech, Moderna, Novavax, Valneva, Janssen and Curevac, as well as a control group. The trial has received ethics approval by the NHS Research Ethics Committee, as well as approval from the Medicines and Healthcare products Regulatory Agency.
The study will be recruiting participants through the NHS COVID-19 Vaccine Research Registry, with vaccinations set to start from the beginning of June.
Participants will be adults aged 30 years or older and will include those immunised early on in the vaccination programme - for example, adults aged 75 and over or health and care workers.
The study will take place at 16 sites across the UK, and will include a total of 2,886 patients. All participants will be monitored throughout the study for any potential side effects and will have bloods taken to measure their immune responses at days 28, 84, 308 and 365, with a small number having additional blood tests at other times. All sites will have an electronic diary for all participants that will send alerts to the team in real-time if needed and a 24-hour emergency phone to a doctor on the study, who can provide further clinical advice.
All the trial sites are working on ways of including people in research from a wide variety of backgrounds and individuals from ethnic minorities are encouraged to apply.
The government is preparing for a potential booster programme based on clinical need and will publish further details in due course. The final policy will be informed by advice from the JCVI and take into account the results of clinical trials.
Professor Saul Faust, Chief Investigator and Director of NIHR Southampton Clinical Research Facility, said: “This trial will give the Joint Committee on Vaccination and Immunisation the important data to inform their recommendations of how to protect the population against any future wave.
“It is fantastic that so many people across the country have taken part in vaccine trials up to now so that we can be in a position to study the effects of boosters, and we hope that as many people as possible over the age of 30 who received their first dose early in the NHS programme will be able to take part.”
Professor Julie Brittenden, Director of Research and Innovation, NHS Greater Glasgow and Clyde, said: “Vaccines are an incredibly important tool for our fight against COVID-19. The team based at our Glasgow Clinical Research have been at the forefront of COVID research over the past year.
“Their work has been vital in developing the vaccines that are now being rolled out across the world. With this study we are supporting further research into the effectiveness of booster vaccines and hope our community will continue to support this vital research.
“Those wishing to take part should sign up to the NHS COVID-19 Vaccine Research Registry and we expect to welcome first patients onto the study in June.”
Professor Emma Thomson, Professor in Infectious Diseases at University of Glasgow Centre for Virus Research, and study lead in Glasgow, said: “This study marks the next step forward in our efforts to understand how to best protect the population and inform future vaccine booster programmes.
“It is the first in the world to provide data on the impact of a third dose and will study seven different vaccines, providing important recommendations for the future.”
Over 2,800 volunteers will be recruited from 18 research sites, including:
- University Hospitals Of Leicester NHS Trust
- Addenbrookes Hospital, Cambridge
- Stockport NHS Foundation Trust, Stepping Hill Hospital
- University Hospitals Sussex
- University College London Hospitals
- Royal Liverpool Hospital
- Northwick Park
- Guy's and St Thomas' NHS Foundation Trust
- Royal Devon and Exeter NHS Foundation Trust
- Oxford Vaccine Group
- University Hospital Southampton NHS Foundation Trust
- Royal Bournemouth Hospital - University Hospitals Dorset
- Portsmouth Hospital
- University Hospitals Birmingham NHS Foundation Trust (UHB)
- NIHR Patient Recruitment Centre Bradford
- Leeds Teaching Hospital
- NHS Greater Glasgow & Clyde Clinical Research Facility
- Public Health Wales - Wrexham

